The next-generation probiotic Akkermansia muciniphila has received the green light from the European Commission as a novel food authorized for use in food supplements and foods for special medical purposes. (1)
1) Akkermansia muciniphila, the next generation probiotic.
Akkermansia muciniphila
was first isolated by Muriel Derrien and Willem de Vos in 2004 from the feces of healthy individuals. Thus, it is a microorganism residing in the gut, and is nevertheless considered a new generation probiotic, already the subject of much research. Its probiotic action is mainly attributed to its ability to strengthen the mucosa and protect the gut barrier (despite its mucin-degrading activity).
1.1) Scientific evidence
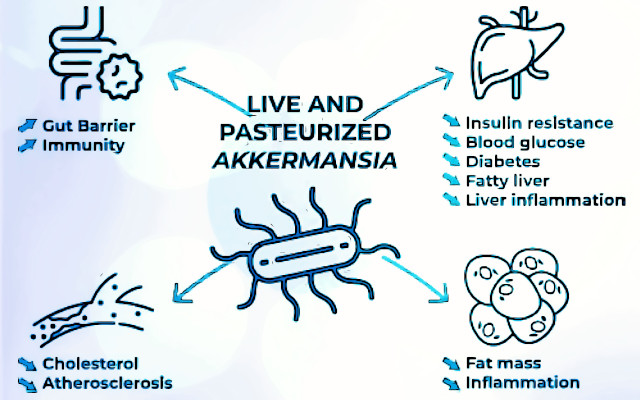
In vivo scientific studies in animals and some clinical trials have shown the efficacy of Akkermansia muciniphila in reducing physiological parameters associated with obesity (e.g., waist circumference, body weight reduction, fat mass). Glucose tolerance and insulin sensitivity are also improved, helping to reduce fat mass and increase lean mass through several diet- or time-dependent effects. These include the anti-inflammatory effect, stimulated by an increase in cytokines.
Intake of live or pasteurized cells of Akkermansia muciniphila is also associated with modulation of the immune, metabolic, and endocannabinoid systems through various metabolites (e.g., short-chain fatty acids, amino acids, and derivatives). Thus, an action on brain function, along thegut-brain axis, is also hypothesized. More evidence is present on the contribution to longevity and health (2,3).
1.2) Synergies with some phytocompounds
Some phytocompounds promote the presence of Akkermansia muciniphila in the intestinal microbiota:
– polyphenols. Phenolic acids, proanthocyanidins, epigallocatechin-3-gallate (EGCG), ellagitannins, anthocyanidins and/or their combination,
– alkaloids. Berberine, betaine, and capsaicin,
– carbohydrates. Dietary fibers, oligofructose, inulin, stachyose, polysaccharides from algae (e.g., fucoidan from brown algae) and microalgae, cyanobacteria (e.g., spirulina),
– traditional Chinese medicine. The wealth of botanicals contained in decoctions and other traditional preparations provides abundance of the above elements. (4)
These phytochemicals – in the context of a balanced diet, such as was seen – contribute to the proper functioning of the gut microbiota through direct stimulation (e.g., production of intestinal mucin, metabolized by the bacterium or growth-promoting factor) and indirect stimulation (e.g., favorable environment for reduction of oxidative stress, reduction of competitive bacterial flora).
2) Akkermansia muciniphila, the Novel Food approval process.
A-Mansia Biotech SA-a Belgian spinoff with a great deal of know-how on Akkermansia muciniphila, based on the work of two professors from Wageningen (NL) and Louvain (B) Universities-had applied for its approval as a novel food on 10/24/19. For the purpose of use as a dietary supplement and ingredient in foods for special medical purposes for adults only, excluding pregnant and lactating women.
2.1) EFSA Evaluation.
EFSA was asked to assess the safety as a novel food of a probiotic from A-Mansia Biotech SA consisting of a pasteurized and freeze-dried bacterial population of Akkermansia muciniphila MucT. Carbohydrates (~45%), protein (~30%), ash (~18%), water (~6%), traces of fat.
EFSA’sNutrition, Novel Foods and Food Allergens (NDA) panel considered the in vivo toxicity study submitted by the applicant as critical, due to which a maximum dose of 3.4 x1010 cells/day could be extrapolated.
The safety of Akkermansia muciniphila was also favorably evaluated with respect to possible risks of allergenicity and acquisition of AMR(Anti-Microbial Resistance) traits. Also taking into account that Akkermansia muciniphila is by its nature resident in the intestine (5,6).
2.2) Novel food authorization with exclusivity
Industrial data protection was recognized by virtue of the numerous studies conducted and submitted in support of the application, such as:
– Bacterial reverse mutation test (Ames test),
– In vitro micronucleus assay with mammalian cells,
– In vivo oral toxicity assay studies in rats over 14 and 90 days,
– toxicity data, (7)
– Flow cytometry validation study,
– antimicrobial resistance (AMR) study.
The European Commission recognized the essential value of these studies for EFSA’s evaluation and therefore granted the applicant a license with exclusivity for a period of 5 years.
3) Interim Conclusions.
The availability of this novel probiotic on the market represents a promising opportunity, in the hope that Akkermansia muciniphila per may contribute to the fight against numerous noncommunicable and chronic-degenerative diseases. (8, 9). Further studies may help understand its mechanisms of action and design more effective and targeted formulations.
Research may also make it possible to identify new strains with enhanced and more specific biological activities that better match the diseases that this probiotic could mitigate. In the hope that interventions to support the microbiota and gut eubiosis can counteract the effects of, among other things, antibiotic resistance.
Dario Dongo and Andrea Adelmo Della Penna
Cover image from https://www.a-mansia.com/
Notes
(1) Reg. EU 2022/168 of the Commission, Authorizing the placing on the market of pasteurized Akkermansia muciniphila as a novel food under Reg. EU 2015/2283 and amending reg. EU 2017/2470. EUR-Lex, http://data.europa.eu/eli/reg_impl/2022/168/oj
(2) Xu et al. (2022). The role of the probiotic Akkermansia muciniphila in brain functions: insights underpinning therapeutic potential. Critical Reviews in Microbiology, https://doi.org/10.1080/1040841X.2022.2044286
(3) Abuqwider et al. (2021). Akkermansia muciniphila, a New Generation of Beneficial Microbiota in Modulating Obesity: A Systematic Review. Microorganims 9:1098, https://doi.org/10.3390/microorganisms9051098
(4) Yue et al. (2022). Dietary strategies to promote the abundance of intestinal Akkermansia muciniphila, a focus on the effect of plant extracts. Journal of Functional Foods 93:105093, https://doi.org/10.1016/j.jff.2022.105093
(5) EFSA, NDA Panel (2021). Safety of pasteurised Akkermansia muciniphila as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 19(9):6780, https://doi.org/10.2903/j.efsa.2021.6780
(6) EFSA’s BIOHAZ panel had already recently evaluated Akkermansia muciniphila as a candidate for the list of biological agents with qualified presumption of safety (QPS. See previous article), taking into account its presence in the colon and human milk. However, uncertainties about the relative abundance of Akkermansia muciniphila in different health and disease conditions (especially in neurology) did not allow the panel to recommend the inclusion of this microorganism in the QPS list. In view of this, the EFSA NDA panel found it necessary to impose among the novel food specifications a number of live cells < 10 CFU/g (the limit of determination)
(7) Druart et al. (2020). Toxicological safety evaluation of pasteurized Akkermansia muciniphila. J. Appl. Toxicol. 41:276–290, https://doi.org//10.1002/jat.4044
(8) Ouyang J, Lin J, Isnard S, Fombuena B, Peng X, Marette A, Routy B, Messaoudene M, Chen Y, Routy JP. (2020). The Bacterium Akkermansia muciniphila: A Sentinel for Gut Permeability and Its Relevance to HIV-Related Inflammation.Front Immunol. 2020 Apr 9;11:645. doi: 10.3389/fimmu.2020.00645. PMID: 32328074; PMCID: PMC7160922
(9) Naito Y, Uchiyama K, Takagi T. A next-generation beneficial microbe: Akkermansia muciniphila. J Clin Biochem Nutr. 2018 Jul;63(1):33-35. doi: 10.3164/jcbn.18-57. Epub 2018 Jun 20. PMID: 30087541; PMCID: PMC6064808.