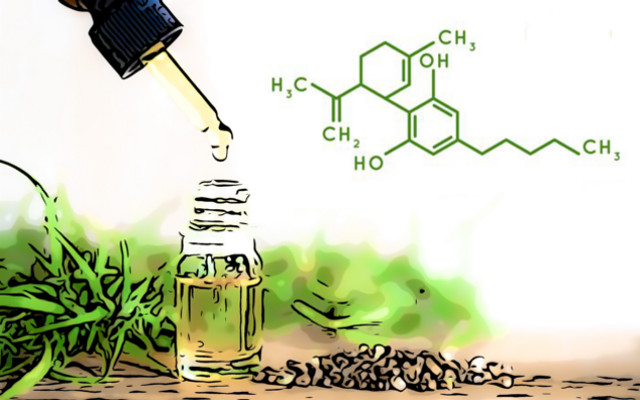
Several applications for the approval of CBD as a novel food are now under consideration by EFSA, with a view to its possible use as an ingredient in food and feed.
The European Commission, it is recalled, finally gave the go-ahead to review the files after recognizing the nonpsychotropic nature of cannabidiol. (1)
Moreover, the favorable outcome of the procedures is not a foregone conclusion, given the strict scientific evidence requirements prescribed by reg. EU 2283/2015. Insight.
CBD, permit applications registered in Brussels
The Novel Foods area of the European Commission’s website indicates only five dossiers related to CBD, cannabidiol. All of the questions in question, however, unfortunately:
– inherent only to synthetic products, and not also to natural CBD (isolated from Cannabis Sativa L.),
– contain confidentiality and data protection clauses designed to ensure exclusive authorization to applicants (i.e., their licensees).
As a result-despite the fact that the substance to be authorized is essentially the same, pure CBD-the other producers will in turn have to submit special applications. With a multiplication of private but also public costs of very dubious utility. (2)
1) Synthetic CBD, PureForm
PureForm Global Inc. and Pureform International have applied for Novel Food approval for a synthetic CBD, made from olivetol and menthadienol. In view of its use in dietary supplements intended for healthy adults at a dosage of 30 mg/day. The summary of the dossier at https://ec.europa.eu/food/system/files/2021-07/novel-food_sum_ongoing-app_2021-2116.pdf
2) Synthetic trans-cannabidiol, CBDepot
CBDepot s.r.o. (Czech Republic) submitted an application for authorization of a synthetic trans-cannabidiol, made through the condensation of olivetol with (1S,4R)-p-mentha2,8-dien-1-ol, under Lewis acid catalysis. The product is then purified by repeated crystallizations, resulting in pure trans-cannabidiol (>99%), free of THC. Intended for adult audiences, average dosage 50 mg/day (0.71 mg/kg body mass).
Summary here.
3) Synthetic CBD, Cibdol AG
Cibdol AG (Switzerland) in turn submitted a dossier for a chemically synthesized cannabidiol, for a dosage up to 150 mg/day. Excluding pregnant and lactating women. The cryptic mirror of information at this link.
4) Synthetic CBD, Chanelle McCoy CBD
Chanelle McCoy CBD LTD (Ireland). Product with high purity (97-102%), intended for adults (excluding pregnant and lactating women in an average dose of 25 mg/day (0.36 mg/kg body mass). In vitro toxicological studies, according to OECD guidelines, to support the safety of the specific product. Summary here.
5) Synthetic CBD, Farmabios
Farmabios S.p.A. (Italy) has in turn applied for approval of a synthetic CBD. Crystalline powder in high purity (98-102%), for use not only in dietary supplements but also in various foods such as nutraceutical beverages (e.g., energy drinks, smoothies and vitamin-enriched juices, and functional drinks), within the threshold most recently recommended in the UK by the Food Standards Agency (FSA). 0.12 mg/kg body mass, equivalent to 4 mg/day for a healthy 70 kg adult). Vulnerable groups excluded. Summary here.
Supporting scientific literature
More than 120 clinical studies cited by Farmabios demonstrate the widespread use of orally administered CBD. No adverse effects, outside of very high dosages (which caused drowsiness, diarrhea, and fatigue in the short term), have been reported.
The literature on the toxicology of CBD is itself very extensive and has, among other things, been the subject of recent reviews by internationally recognized scientific authorities. The same applies to pharmacokinetics and toxicokinetic parameters (T1/2, AUC, bioavailability, Cma* and Tmax).
CBD, more dossiers under review by EFSA.
CBD-related files are far more numerous than the meager list published on the European Commission website. In fact, the EFSA(European Food Safety Authority) portal reports to a wide range of inquiries that include natural CBD.
Of particular note in the latter regard are the dossiers submitted by Linnea S.A (NF 2020/2245, application registered 12.7.21) and HemPoland (NF 2020/2230, 10.6.21). In addition to those registered under NF codes 2020/1696 (21.6.21) and NF 2021/2340 (11.5.21).
One Channel Away
Hemp farmers and hemp processing laboratories in the European Union continue to battle the windmills of Brussels bureaucracy, in the still hollow hope that they will be able to claim authorization for the use of natural CBD as an ingredient in supplements and foods. Without having to pay the duty of licensing, it is hoped, to the pioneers of EU procedures who obtain exclusives based on irrelevant confidentialities or open secrets. (2)
In the UK freed from Brussels bureaucrats, meanwhile, healthy companies continue marketing CBD for food use under a transitional regime, serenely awaiting the approvals to come. (4) From one side of the Channel to the other, stagnation and uncertainty or growth with the wind in its sails and competitiveness in international markets in a still promising sector. Will it be okay or will it be better to correct the procedures, before everyone’s exodus to the UK?
Dario Dongo
Notes
(1) Dario Dongo, Silvia Giordanengo. Cannabis Sativa, CBD. Green light from UN and European Commission. GIFT (Great Italian Food Trade). 7.12.20, https://www.greatitalianfoodtrade.it/progresso/cannabis-sativa-cbd-via-libera-da-onu-e-commissione-europea
(2) The very extensive literature supporting the food consumption safety of CBD should therefore justify the authorization of the substance regardless of the in vitro toxicological studies on individual products, moreover made by the same processes with few exceptions. The approach adopted by the European institutions, conversely, involves the granting of exclusivity to individual applicants its sole basis of instances of ‘confidentiality’ and ‘data protection’ that in several cases completely disregard the specifics of production (e.g., patents) or the relevance of the studies they commission with respect to the scientific safety assessment of the Novel Food
(3) Novel Food authorization of CBD in the UK? Attorney Dario Dongo replies. FARE(Food and Agriculture Requirements). 7/31/21, https://www.foodagriculturerequirements.com/archivio-notizie/domande-e-risposte/autorizzazione-novel-food-del-cbd-in-uk-risponde-l-avvocato-dario-dongo
Dario Dongo, lawyer and journalist, PhD in international food law, founder of WIISE (FARE - GIFT - Food Times) and Égalité.